What Is The Molal Boiling Point Elevation Constant The molal elevation constant is the elevation in the boiling point of a solvent when one mole of a non volatile solute is dissolved in it per kilogram of solvent
Assertion One molal aqueous solution of glucose contains 180 g of glucose in 1 kg water Reason The solution containing one mole of solute in 1000 g of solvent is called one molal Molal elevation constant may be defined as the elevation in boiling point when one mole of solute is dissolved in 1000 grams of the solvent
What Is The Molal Boiling Point Elevation Constant

What Is The Molal Boiling Point Elevation Constant
https://i.ytimg.com/vi/gXnCrWS7IzQ/maxresdefault.jpg

Calculating Molar Mass From Boiling Point Elevation YouTube
https://i.ytimg.com/vi/WXaBRSYLgdU/maxresdefault.jpg

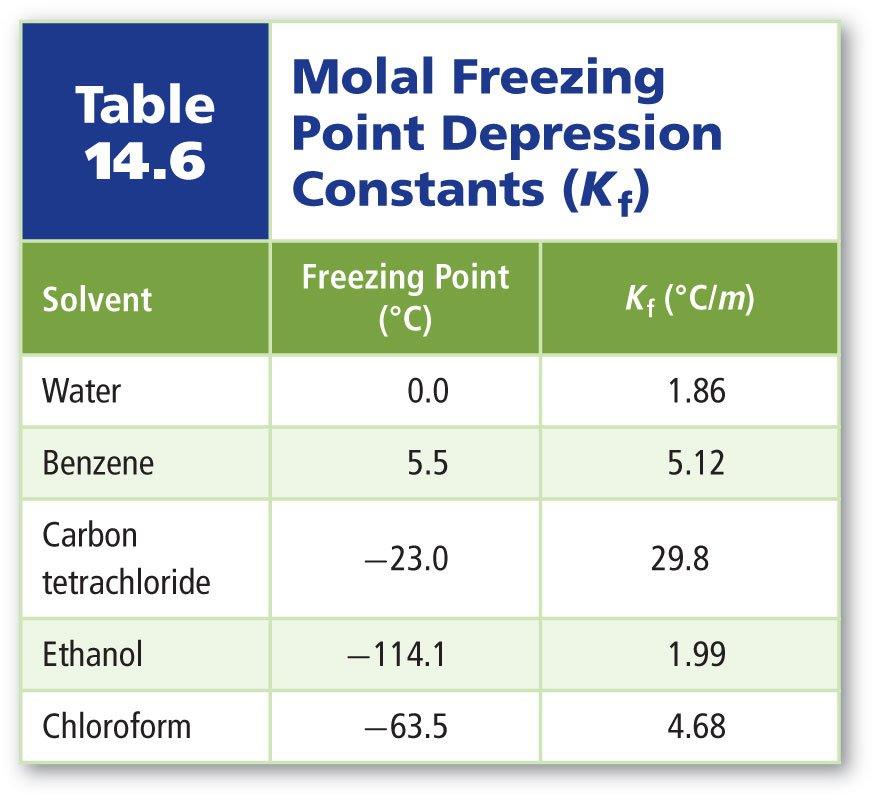
Calculate The Molal Elevation Constant K b For Water And The
https://i.ytimg.com/vi/ioRvSd2np2E/maxresdefault.jpg
Calculate the vapour pressure of an aqueous solution of 1 0 molal glucose solution at 100oC A solution of 2 5 g of a non volatile solid in 100 g benzene is boiled at 0 42 K higher than the boiling point of pure benzene Calculate the molar mass of the substance Molal elevation
Elevation in the boiling point for 1 molal solution of glucose is 2K The depression in the freezing point of 2 molal solutions of glucose in the same solvent is 2K The molal depression constant for water is 1 86oC The freezing point of a 0 05 molal solution of a non electrolyte in water is
More picture related to What Is The Molal Boiling Point Elevation Constant

ALEKS Using A Solution Freezing Point To Calculate A Molar Mass YouTube
https://i.ytimg.com/vi/0yop8BYvV10/maxresdefault.jpg

Explain Elevation In Boiling Point And Derive Equation For Molal
https://i.ytimg.com/vi/WA-UbQdd5UQ/maxresdefault.jpg

CHEM 201 Calculating Boiling Point Of A Non Electrolyte Solution YouTube
https://i.ytimg.com/vi/jk8XXgYsK08/maxresdefault.jpg
a Define the following terms i Molarity ii Molal elevation constant Kb b A solution containing 15 g urea molar mass 60 g mol 1 per litre of solution in water has the same 1000g of 1 molal aqueous solution of sucrose is cooled and maintained at 3 534oC Find out how much ice will separate out this temperature Kf for water 1 86k m 1
[desc-10] [desc-11]
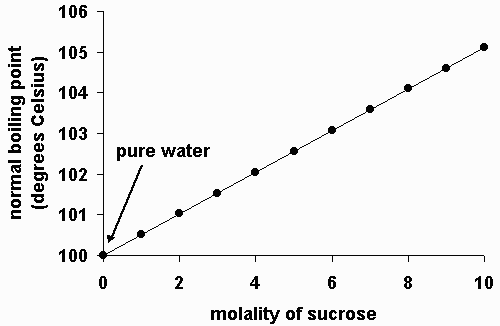
Boiling Point Elevation
https://www.chem.purdue.edu/gchelp/solutions/watsuc2.gif
Colligative Properties pptx On Emaze
http://userscontent2.emaze.com/images/98d490ea-020e-41a5-9584-e08038c432fd/81292f1e-c317-4637-8c27-fde9531fa5b5image20.jpeg

https://www.toppr.com › ask › question › what-is-molal-elevation-constan…
The molal elevation constant is the elevation in the boiling point of a solvent when one mole of a non volatile solute is dissolved in it per kilogram of solvent

https://www.toppr.com › ask › question
Assertion One molal aqueous solution of glucose contains 180 g of glucose in 1 kg water Reason The solution containing one mole of solute in 1000 g of solvent is called one molal

The Molal Elevation Constant Of Water Is 0 56 K Kg Mol the Boiling

Boiling Point Elevation

The Molal Elevation Constant Of Water Is 0 56 K Kg Mol the Boiling

Boiling Point Elevation Chemistry Steps
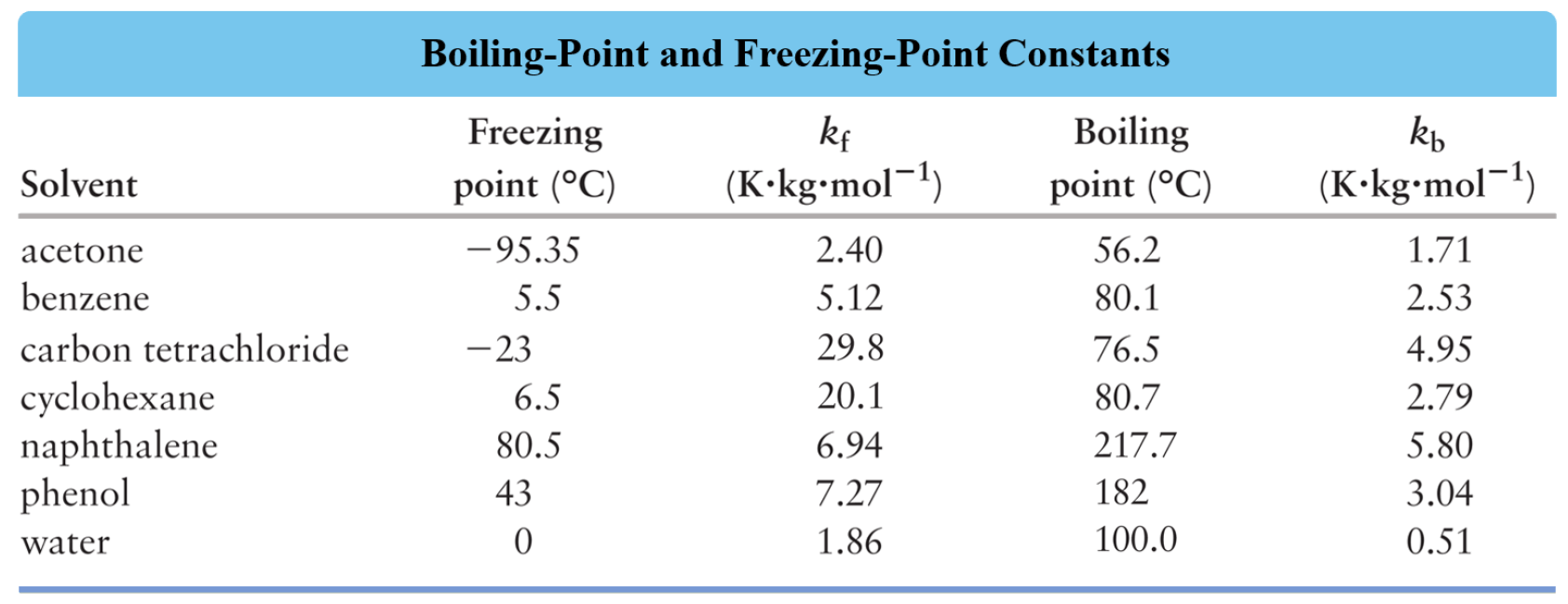
Boiling Point Elevation Chemistry Steps
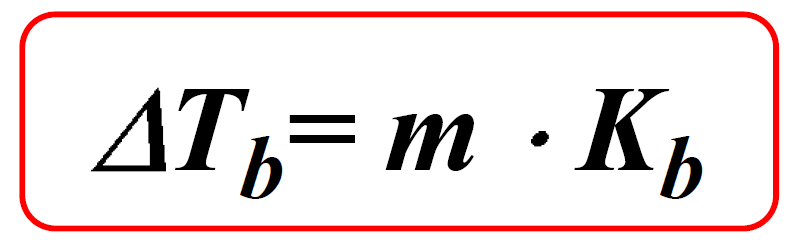
Boiling Point Elevation Chemistry Steps
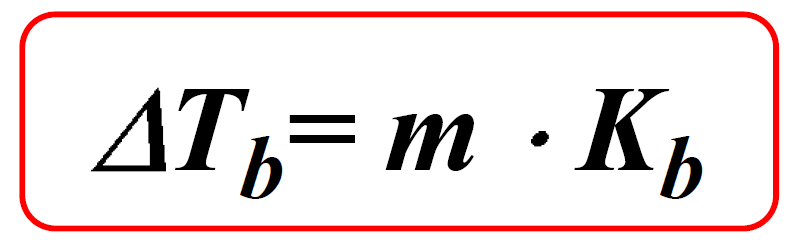
Boiling Point Elevation Chemistry Steps
.PNG)
Chemical Equation For Water Boiling Tessshebaylo

The 54th One The Molal Elevation Constant Of Water Is 0 51 The
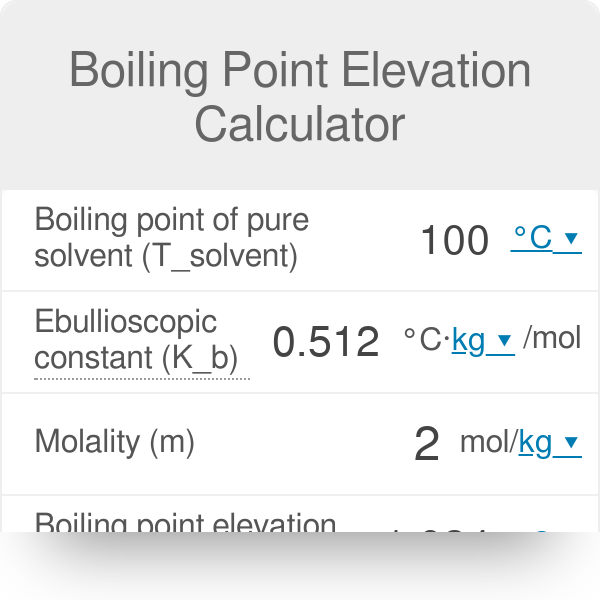
How To Find Elevation Of Boiling Point Tutorial Pics
What Is The Molal Boiling Point Elevation Constant - A solution of 2 5 g of a non volatile solid in 100 g benzene is boiled at 0 42 K higher than the boiling point of pure benzene Calculate the molar mass of the substance Molal elevation