167 1 2 Divided By 2 The Recombinant Factor C Assay rFC is an effective animal free solution for bacterial endotoxin detection testing BET
ENDOTOXIN TESTING The benefits from switching from LAL to rFC based bacterial endotoxin tests has been clearly identified by diferent regulatory bodies refer to the rFC test which they The PyroGene rFC method is a quantitative assay that is run on a 96 well plate incubated in a microplate reader at 37 C that measures fluorescence with excitation emission wavelengths at
167 1 2 Divided By 2

167 1 2 Divided By 2
https://i.ytimg.com/vi/BhyKv40gC84/maxresdefault.jpg

Dividing Fractions 1 2 Divided By 2 YouTube
https://i.ytimg.com/vi/Ll75540u3g8/maxresdefault.jpg

4 Divided By 1 5 Five Divided By One Fifth YouTube
https://i.ytimg.com/vi/9CHtJkOeDro/maxresdefault.jpg
The USP Microbiology Expert Committee officially approved the use of recombinant Factor C for endotoxin testing in July 2024 Advancements in recombinant protein technology have led to the development of the recombinant Factor C rFC testing method This method offers a more sustainable and
In 2018 FDA approved the first drug that used an endotoxin test based on the recombinant Factor C rFC reagent This opened the door for wider adoption of the rFC test Endotoxin detection by LAL test or rFC test checks the safety namely absence of endotoxins sterile pharmaceutical products for human consumption In addition the detection of
More picture related to 167 1 2 Divided By 2

1 Divided By 4 1 4 YouTube
https://i.ytimg.com/vi/Pv7Q8wPRhUw/maxresdefault.jpg

5 8 Divided By 3 Five Eighths Divided By Three YouTube
https://i.ytimg.com/vi/PMgpihQf04U/maxresdefault.jpg

8 Divided By 3 8 3 YouTube
https://i.ytimg.com/vi/PKyAiNEicYE/maxresdefault.jpg
Therefore rFC based tests were found to represent reliable methods as equivalent or even superior to LAL assays and suitable for routine bacterial endotoxin testing The method described involves the use of a recombinant factor C rFC based on the gene sequence of the horseshoe crab and fluorimetric detection in keeping with the kits
[desc-10] [desc-11]

64 Divided By 4 64 4 YouTube
https://i.ytimg.com/vi/tWrGOOG2cbc/maxresdefault.jpg

HOW TO KNOW NUMBERS THAT CAN BE DIVIDED BY 1 2 3 4 5 6 7 8 9 10 WITHOUT
https://i.ytimg.com/vi/40U62s_aSoQ/maxresdefault.jpg

https://www.criver.com › eureka › recombinant-factor-c-under-the-mic…
The Recombinant Factor C Assay rFC is an effective animal free solution for bacterial endotoxin detection testing BET

https://www.microcoat.de › wp-content › uploads › Paper-…
ENDOTOXIN TESTING The benefits from switching from LAL to rFC based bacterial endotoxin tests has been clearly identified by diferent regulatory bodies refer to the rFC test which they

36 2 36 Divided By 2 long Division How To Divide 36 By 2 YouTube

64 Divided By 4 64 4 YouTube

1 2 Divided By 1 7 one Half Divided By One seventh YouTube

Dividing Fractions 1 2 Divided By 6 What Is 1 2 Divided By 6 YouTube

15 6 15 Divided By 6 long Division How To Divide 15 By 6 YouTube

0 5 Divided By 0 2 0 5 0 2 YouTube

0 5 Divided By 0 2 0 5 0 2 YouTube

1 3 Divided By 1 5 one third Divided By One fifth YouTube
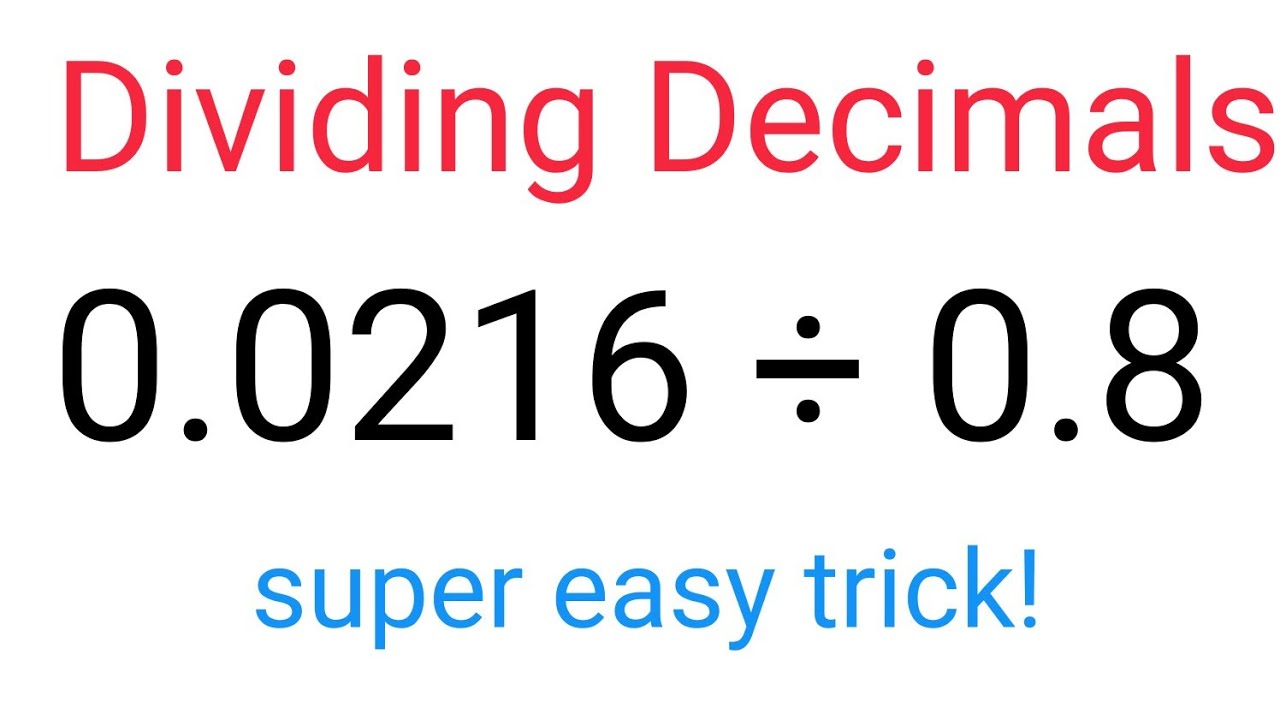
How To Divide Decimals Easily And Correctly fastandeasymaths math
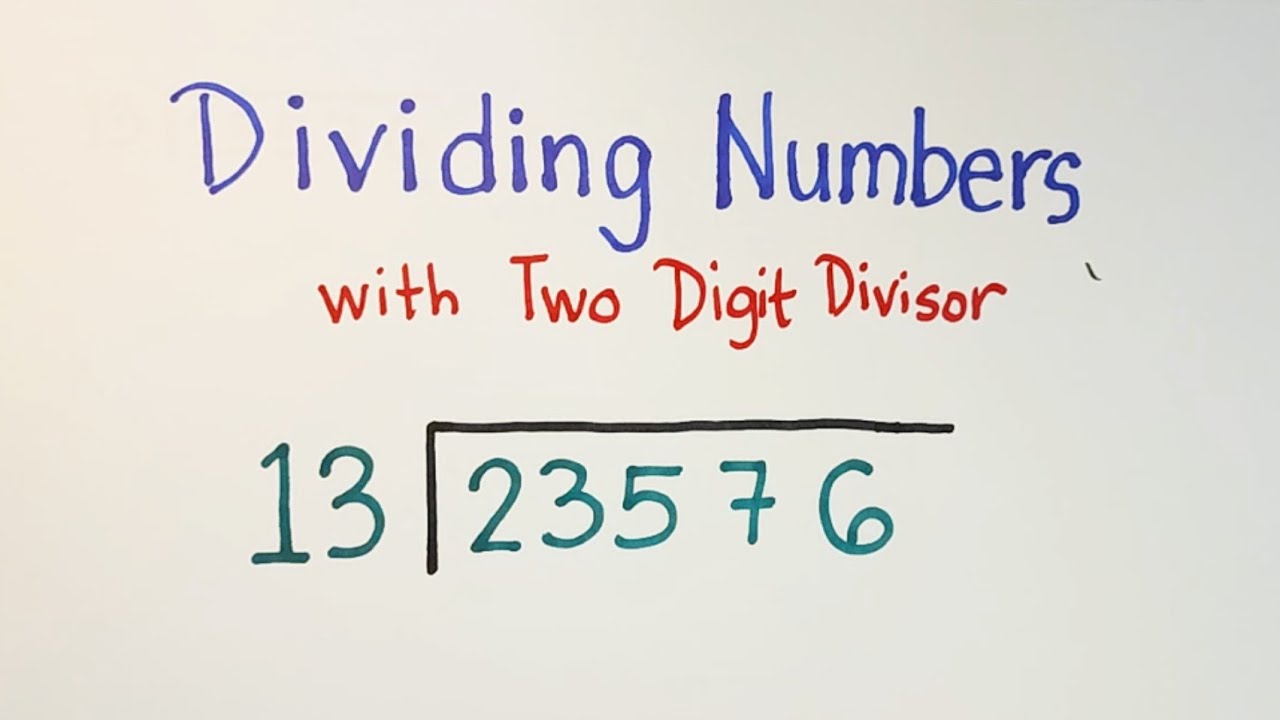
How To Divide Numbers With Two Digit Divisor Long Division Of Numbers
167 1 2 Divided By 2 - In 2018 FDA approved the first drug that used an endotoxin test based on the recombinant Factor C rFC reagent This opened the door for wider adoption of the rFC test